Answer:
The temperature change is 7.2 °C
Step-by-step explanation:
Let the temperature change be given by 'x'.
Given:
Mass of water is,
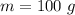
Heat lost by water is,
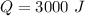
Specific heat capacity of water is,
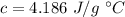
We know that, heat energy lost by 'm' g of water for a temperature change of 'x' °C is given as:
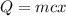
Rewriting the above in terms of 'x', we have
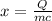
Plug in 100 for 'm, 4.186 for 'c', 3000 for 'Q' and solve for 'x'. This gives,

Therefore, the temperature change is 7.2 °C.