Answer:
The mass of the kerosene is,
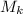 = 0.021 kg
= 0.021 kg
Step-by-step explanation:
Given data,
The mass of the empty density bottle, m₁ = 0.03 kg
The mass of density bottle filled with water, m₂ = 0.057 kg
The relative density of kerosene is, ρ = 0.8
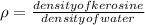
Density of kerosene = Density of water x relative density
= 1000 kg/m³ x 0.8
= 800 kg/m³
The mass of water in the bottle,
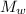 = m₂ - m₁
= m₂ - m₁
= 0.057 kg - 0.03 kg
= 0.027 kg
The volume of bottle,

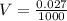
V = 2.7 x 10⁻⁵ m³
The mass of the kerosene,

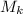 = 800 x 2.7 x 10⁻⁵
= 800 x 2.7 x 10⁻⁵
= 0.021 kg
Hence, the mass of the kerosene is,
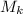 = 0.021 kg
= 0.021 kg