Answer:
Volume occupied by Neon gas is 52.67 L
Step-by-step explanation:
Using Ideal Gas Equation:
PV = nRT
where
P = pressure exerted by the gas = 57 atm
V = volume occupied = ?
n = number of moles = 115 moles
R = Ideal gas constant = 0.0821 L.atm/K.mol
(R value should be taken according to the units of Temperature,pressure, volume and mole)
T = Temperature = 45 + 273 = 318 K
(For temperature conversion from C to K add 273 to temperature:T + 273)
PV = nRT , So
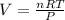
Put values of T,P,n,R
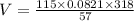
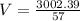
V = 52.67 L
Volume occupied by 115 moles of Neon gas at 57 atm Pressure and 45 C temperature is 52.67 L