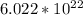Answer:
Step-by-step explanation:
- 1 mole of any substance contains Avogadro number of particles i.e, 6.022 × 10^23 particles.
- number of of particles in n moles of a substance = n*Avogadro number
- number of moles of Ne in 2.0 g of Ne= 2/20=0.1
[number of moles=given weight/ gram molecular weight]
- so, number of particles in 2.0 g of Ne = number of particles in 0.1 moles of Ne= 0.1(6.022 × 10^23)
=