Answer:
Density of water is 1 g/cm³.
Step-by-step explanation:
Given:
Mass of given volume of water is,
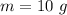
Volume of the given amount of water is,
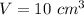
We know that, density of a quantity is given as the ratio of its mass to that of its volume.
Here, in order to calculate density of water, we need to divide the mass of water by its volume. Therefore,
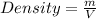
Plug in 10 g for 'm', 10 cm³ for 'V and solve for density. This gives,
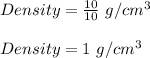
Therefore, the density of water of mass 10 g and volume 10 cm³ is 1 g/cm³.