Answer:
The force between them is, F = 8.33 x 10⁻¹¹ N
Step-by-step explanation:
Given data,
The distance between the proton and electron, x = 12 nm
= 12 x 10⁻⁹ m
The electrostatic force between them is given by the formula,
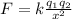
Where k = 9 x 10⁹ Nm²C⁻²
Substituting the given values,
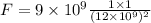
F = 8.33 x 10⁻¹¹ N
Hence, the force between them is, F = 8.33 x 10⁻¹¹ N