Answer:
When electron drops from n=6 to n=2, it emits wavelength of 410 nm which gives violet color.
Step-by-step explanation:
Using Rydberg's Equation:
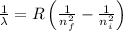
Here
R= Rydberg's constant =
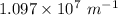
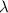 = Wavelength of photon emitted
= Wavelength of photon emitted
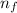 = 2
= 2
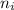 = 6
= 6
Put the values in the formula:
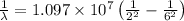
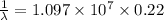
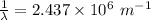
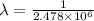
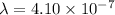 m
m
 = 410 nm
= 410 nm
(1 m =
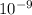 )
)
This wavelength( 410 nm) is emitted by VIOLET color