Answer:
The mass of sugar used is 427.5 grams.
Step-by-step explanation:
The formula of common sugar is
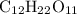 .
.
Its molar mass of molecule is 342 grams per mole.
The number of moles of sugar used is equal to '1.25'
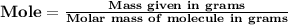
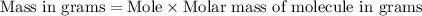
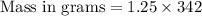
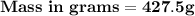
(NOTE : You can calculate molar mass by adding the mass of all the atoms present in the atom.
Mass of Oxygen (O) = 16 ; Total number of Oxygen atoms present = 11
Mass of Hydrogen (H) =1 ; Total number of Hydrogen Atoms present = 22
Mass of Carbon (C) = 12 ; Total number of Carbon atoms present = 12
Total mass of molecule = 12 × 12 + 1 × 22 + 16 × 11 = 342)