Answer:
The approximate bond angle for a molecule with a linear shape is
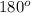
Step-by-step explanation:
Linear shape is the basic shape of the organic molecules. All diatomic molecules has linear shape. These two atoms are bonded together by either single,double or triple bond.
For example, HCl has singe bond.
Some tri atomic molecules are also be linear.For example
 .
.
In the shape of carbondioxide the central atom is carbon and it hare four valence electrons to the each oxygen atom.And make double bond with each oxygen atom.
The shape of the molecule is linear and bond angle is
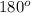 .
.