Answer:
a) unstable
b) unstable
c) unstable
d) Stable
Step-by-step explanation:
a)
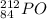
The given isotope , atomic number is greater than 83.(84>83).
Therefore, it is unstable.
b)
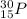
The given isotope has even number of protons(15) and neutrons(15).
Therefore, it is unstable.
c)
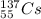
The given isotope has even number of protons(15).
Therefore, it is unstable.
d)
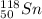
The given isotope have even number of neutrons ( 68) an d the proton ratio is
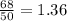 .It is more than 1.25.
.It is more than 1.25.
Therefore, it is stable.