Answer:
temperature and mechanism
Step-by-step explanation:
Arrhenius equation gives us the idea about the factors affecting equilibrium constant.It is given as:
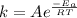
where k=equilibrium constant
A=pre-exponential factor
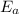 =Activation energy
=Activation energy
T=temperature
R=Universal gas constant
It is clear that k depends only on temperature and activation energy.
Activation energy of a reaction is different for different mechanisms or pathways.Hence k depends on both temperature and mechanism.