Answer: The total pressure of container at equilibrium is 0.431 bar
Step-by-step explanation:
We are given:
Pressure of hydrogen sulfide = 0.240 bar
The given chemical equation follows:
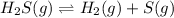
Initial: 0.240
At eqllm: 0.240-x x x
The expression of
 for above equation follows:
for above equation follows:
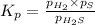
We are given:
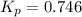
Putting values in above expression, we get:
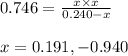
Neglecting the negative value of 'x' because pressure cannot be negative.
So, the equilibrium pressure of hydrogen gas = x = 0.191 bar
The equilibrium pressure of sulfur gas = x = 0.191 bar
The equilibrium pressure of hydrogen sulfide gas = (0.240 - x) = (0.240 - 0.191) = 0.049 bar
Total pressure of the container at equilibrium =
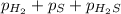
Total pressure of the container at equilibrium = 0.191 + 0.191 + 0.049 = 0.431 bar
Hence, the total pressure of container at equilibrium is 0.431 bar