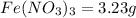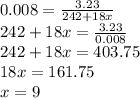Answer:
The empirical formula is :
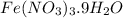
Step-by-step explanation:
Let us assume the moles of hydration in ferric nitrate is '
 '
'
Thus the formula of the compound becomes :
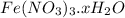
Now when its heated , the reaction proceeds like :
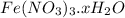 ⇒
⇒
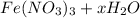
Given ,
- The mass of anhydrated
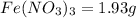
Molar mass of
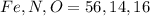 respectively.
respectively.
The mass of 1 mol of
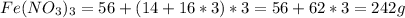
The number of moles =
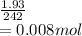
Therefore the number of hydrated moles must also be 0.008
Given ,
- The mass of hydrated