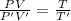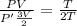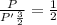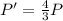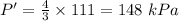Answer:
The final pressure is 148 kPa
Solution:
As per the question:
Initial Pressure of the gas, P = 111 kPa
Initial Volume, V = 5.00 l
Now,
In order to calculate the final pressure of the system:
At constant pressure, the work done is 'W'
If the absolute initial temperature be T
When the absolute temperature is doubled, T' =2T
Total work done in the processes, W =
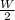
Now,
From the ideal gas eqn:
PV = nRT
PV ∝ T (1)
In constant pressure process, work done is given by:
W =
 (2)
(2)
Thus from eqn (1) and (2):
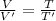
In the first process, temperature is doubled:
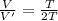
V = 2V'
Thus
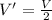
In the process, the change in volume is
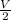 given by:
given by:
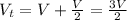
Now,