Answer:
Maximum safe operating temperature = 370°C
Step-by-step explanation:
Radius of stainless steel vessel = 25.0/2 = 12.5 cm
height of vessel = 30.0 cm
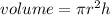
Substitute the values in the above formula
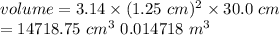
Maximum safe temperature is calculated by using ideal gas equation
PV = nRT
pressure, P = 6.60 mPa =
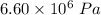
R = 8.314 J/mol.K
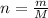
molecular mass of CO2 = 44 g/mol
mass of CO2 = 0.800 kg = 800 g
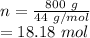
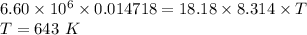
T (K)=T (°C) + 273.15
643 K = T (°C) + 273.15
T = 643 - 273.15
= 369.85 °C = 370°C
Maximum safe temperature = 370°C