Answer:
Molarity of potassium chloride is:
A. 1.0 M
Step-by-step explanation:
Molarity is used to express the concentration of the solution.It is defined as the moles of solutes per liter volume of solution.It is denoted by M.
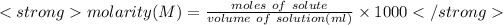
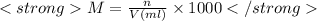
Here'n' is the number of moles. It can be calculated using formula:
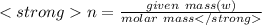
given mass=30 gm
Molar mass of KCl = mass of K+mass of Cl
= 39.098+34.45
= 74.55 g
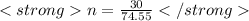
n = 0.402 moles
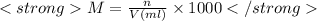
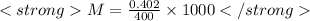
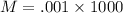
M=1.00 M
So molarity of potassium chloride is 1.00 M