Answer:
The mass of ammonia gas is 36.4 grams.
Step-by-step explanation:
Ammonia gas combines with excess oxygen gas to produce nitric oxide and water.
The chemical reaction is as follows.
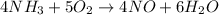
The
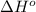 of the reaction is -1170 kJ.
of the reaction is -1170 kJ.
When 363 kJ energy is released .
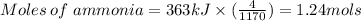
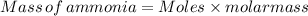
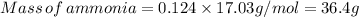
Therefore, The mass of ammonia gas is 36.4 grams.