Answer:
The water lost is 463.95 g.
Step-by-step explanation:
Given that,
Heat lost rate = 657 W
Time = 28.6 min
Heat vaporization = 2430 J/g
We need to calculate the heat lost
Using formula of heat loss
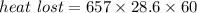
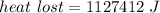
We need to calculate the water lost
Using formula of water lost
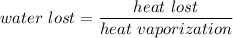
Put the value into the formula
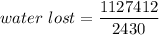
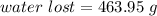
Hence, The water lost is 463.95 g.