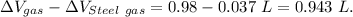Answer:
Volume of gasoline spills out is 0.943 L.
Step-by-step explanation:
Volumetric expansion of both gasoline and steel tank is :
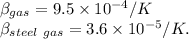 { source Internet}
{ source Internet}
We know expansion due to temperature change is :
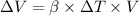
For gasoline:
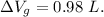
Similarly for Steel tank:
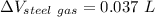 .
.
Now, volume of gasoline spills out is equal to difference between expansion in volume.