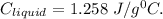Answer:
Specific heat of liquid
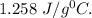
Step-by-step explanation:
We know in thermal equilibrium :
Loss in heat by iron block = Gain in heat by liquid .
Specific heat of iron = 0.45
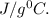 { source internet }
{ source internet }
Now , loss in heat by iron block =
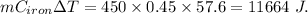
Heat gain by liquid=
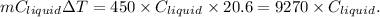
Equating both we get :