Answer:
The maximum efficiency of this thermodynamic engine is η=0.78
Step-by-step explanation:
The maximum efficiency of a thermodynamic engine is consequence of the Maximum Work Theorem, and corresponds to a Carnot Cycle.
The expression is given by
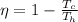
where
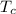 is the temperature of the cold reservoir, and
is the temperature of the cold reservoir, and
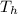 is the temperature of the hot reservoir, therefore
is the temperature of the hot reservoir, therefore
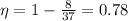
is the maximum efficiency of this thermodynamic engine.
* It should be noted that a Carnot Cycle is not possible in real engines, it is a theoric tool that allows the development of the thermodynamic engines.