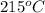Answer : The temperature for non-catalyzed reaction will be,
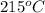
Explanation :
Activation energy : The energy required to initiate the reaction is known as activation energy.
According to the Arrhenius equation,
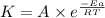
Since, the rate for both the reaction are equal.
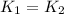
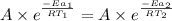
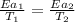 ..........(1)
..........(1)
where,
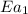 = activation energy for non-catalyzed reaction = 70.0 kJ/mol
= activation energy for non-catalyzed reaction = 70.0 kJ/mol
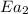 = activation energy for catalyzed reaction = 42.0 kJ/mol
= activation energy for catalyzed reaction = 42.0 kJ/mol
 = temperature for non-catalyzed reaction = ?
= temperature for non-catalyzed reaction = ?
 = temperature for catalyzed reaction =
= temperature for catalyzed reaction =
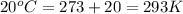
Now put all the given values in the above formula 1, we get:
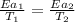
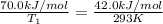
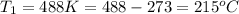
Therefore, the temperature for non-catalyzed reaction will be,