Answer:
Percentage of mass of solute in aqueous phase is 28.57%.
Step-by-step explanation:
 - Fraction of solute in aqueous phase.
- Fraction of solute in aqueous phase.
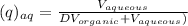
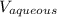 = Used volume of aqueous solution
= Used volume of aqueous solution
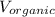 = Used volume of organic solution
= Used volume of organic solution
D = partition coefficient.
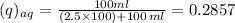
Mass of solute in aqueous phase =
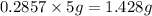
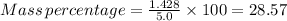
Therefore, Percentage of mass of solute in aqueous phase is 28.57%.