The question is incomplete, here is a complete question.
An arctic weather balloon is filled with 27.8 L of helium gas inside a prep shed. The temperature inside the shed is 13 ⁰C. The balloon is then taken outside, where the temperature is -9 ⁰C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits.
Answer : The new volume of the balloon is 25.7 L
Explanation :
Charles's Law : It is defined as the volume of the gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
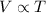
or,
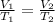
where,
 = initial volume of gas = 27.8 L
= initial volume of gas = 27.8 L
 = final volume of gas = ?
= final volume of gas = ?
 = initial temperature of gas =
= initial temperature of gas =
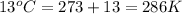
 = final temperature of gas =
= final temperature of gas =
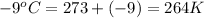
Now put all the given values in the above equation, we get:
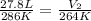
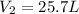
Therefore, the new volume of the balloon is 25.7 L