Answer:
(A) Th = 818.6 K
(B) Qh = 14211.7 J
Step-by-step explanation:
efficiency (n) = 0.537
temperature of cold reservoir (Tc) = 379 K
heat rejected (Qc) = 6580 J
(A) find the temperature of the hot reservoir (Th)
n = 1 -

0.537 = 1 -
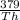
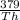 = 1 - 0.537 = 0.463
= 1 - 0.537 = 0.463
Th =
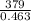
Th = 818.6 K
(B) what amount of heat is put into the engine (Qh) ?
from

Qh = 6580 ÷

Qh = 14211.7 J