Answer:
1658.01807 cm³
Step-by-step explanation:
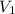 = Initial volume = 640 cm³
= Initial volume = 640 cm³
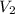 = Final volume
= Final volume
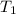 = Initial temperature = 20°C
= Initial temperature = 20°C
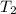 = Final temperature = -20°C
= Final temperature = -20°C
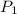 = Initial pressure = 1 atm (sea level)
= Initial pressure = 1 atm (sea level)
Final pressure
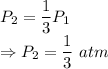
We have the relation
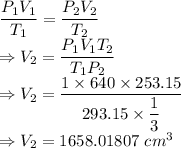
The final volume is 1658.01807 cm³