Answer:
178.0 kJ
Step-by-step explanation:
In order to calculate the enthalpy change for a reaction, we should know the enthalpy of formation values for each component present in the chemical equation. This means we should use a trusted source containing a table of enthalpy of formation values.
Given:
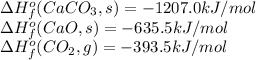
Knowing this, the enthalpy change for a reaction is calculated by summing all the enthalpy values of formation of the products (multiplied by the stoichiometric coefficients of each) and subtracting the sum of enthalpy values of formation of the reactants (multiplied by the stoichiometric coefficients as well):
![\Delta H^o_r=[1 mol\cdot \Delta H^o_f(CaO, s)+1 mol \cdot \Delta H^o_f(CO_2, g)]- 1 mol\cdot \Delta H^o_f (CaCO_3, s)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/dvxovs4wfk23sof1328x5hah729fauti49.png)
Substitute the given values:
![\Delta H^o_f(CaCO_3, s)=-1207.0 kJ/mol\\\Delta H^o_f(CaO, s)=-635.5 kJ/mol\\\Delta H^o_f(CO_2, g)=-393.5 kJ/mol\\\Delta H^o_r = [1 mol\cdot (-635.5 kJ/mol) + 1 mol\cdot (-393.5 kJ/mol)] - 1 mol\cdot (-1207.0 kJ/mol) = 178.0 kJ](https://img.qammunity.org/2020/formulas/chemistry/middle-school/mnc6y3sduo7l0f3uxehn8y3nv8zzu1ogka.png)