Answer:
(a). The first wavelength is 401.0 nm.
(b). The metal's work function is 2.55 eV.
Step-by-step explanation:
Given that,
Maximum kinetic energy = 0.65 eV
Second wavelength
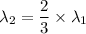
(a). We need to calculate the wavelength
Using equation of work function for first wavelength
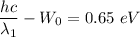 .....(I)
.....(I)
For second wavelength,
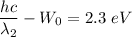
Put the value of second wavelength
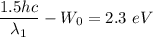 ....(II)
....(II)
By subtraction equation (I) from (II)
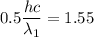
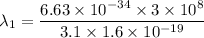
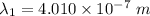
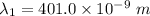
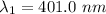
(b). We need to calculate the work function
Using formula of work function
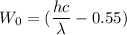
Put the value into the formula
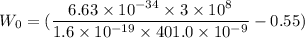
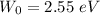
Hence, (a). The first wavelength is 401.0 nm.
(b). The metal's work function is 2.55 eV.