Answer:
0.196 M
Step-by-step explanation:
First, we have to calculate the moles of sodium hydroxide.
moles = mass / molar mass
moles = 3.9280 g / (39.997 g/mol) = 0.098207 mol
It is important to realize that even though 300 mL is the volume of water firstly added to dissolve the hydroxide, the flask is then filled to the mark, meaning that the volume of the solution is 500 mL = 0.500 L.
The molar concentration of sodium hydroxide is:
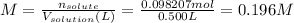
The concentration is an intensive property, that is, it does not depend on the amount of matter. As a consequence, the concentration of the 50 mL of solution in the buret is the same as the concentration in the flask.