Answer:
T = 457.2 K
Step-by-step explanation:
mass of balloon (Mb) = 295 kg
pressure of outside air (Pa) = 1.01 x 10^{5}
density of air (ρ) = 1.29 kg/m^{3}
volume (v) = 568 m^{3}
molecular mass of air (m) = 29 u
F = mg = ρvg
(Mb + Ma)g = ρvg
(Mb + Ma) = ρv
295 + Ma = 1.29 x 568
Ma = 437.72 kg = 437.72 x 10^{3} g
number of moles (n) = mass / molar mass
number of moles (n) = 437.72 x 10^{3} / 29 = 15,093.8 moles
from the ideal gas law pv = nRT
T =
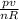
T =
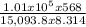
T = 457.2 K