Answer:
1°C temperature change will be observed if a sample of 100 g of ethylene glycol antifreeze solution.
Step-by-step explanation:
Mass of ethylene glycol = m = 100 g
Specific heat capacity of ethylene glycol = c = 3.5 J/g°C
Change in temperature of ethylene glycol = ΔT
Heat loss by the ethylene glycol = Q = 350 J
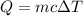

ΔT = 1°C
1°C temperature change will be observed if a sample of 100 g of ethylene glycol antifreeze solution.