Answer:
Correct option -D
Step-by-step explanation:
Here, A kinetics experiment is set up is set up to collect the gas that is generated from the cacarbonate and methanoic acid.
Among the given conditions, decreasing the particle size of calcium carbonate only increases the production of gas.
Smaller particles of reactant increases the surface area then followed by rate of reaction will be increases it leads to increases the production of gas.
Therefore, the suitable experimental condition most likely to increase the gas production is-
Decreasing the particle size of the
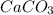 by grinding it into a fine powder.
by grinding it into a fine powder.
Hence, correct option -D.