Answer:
230
Step-by-step explanation:
Given equilibrium conditions, if a system is at equilibrium, this means the rate of a forward reaction becomes equal to the rate of a reverse reaction and the concentrations of each species become constant (stop to change).
This is defined by the equilibrium constant which defines the concentrations at equilibrium. For the given reaction, the equilibrium constant can be described as the ratio between the concentrations of products (raised to the power of their coefficients) and the concentrations of reactants (raised to the power of their coefficients):
![K_(eq)=([N_2][H_2]^3)/([NH_3]^2)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/zqwbtvpk31s9xohfu3ioehuq841o1ox9gc.png)
Q, on the other hand, is a reaction quotient. It is used only to determine where the equilibrium will shift when the system is actually not at equilibrium. The expression or Q is exactly same, but concentrations used would be non-equilibrium concentrations. There are three cases:
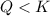 : in this case, equilibrium shifts towards the formation of products;
: in this case, equilibrium shifts towards the formation of products;
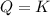 : the system is at equilibrium;
: the system is at equilibrium;
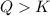 : equilibrium shifts towards the formation of reactants.
: equilibrium shifts towards the formation of reactants.
Looking at the context of our problem, since this system is at equilibrium, then Q = K = 230.