Answer:
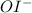
Step-by-step explanation:
An intermediate is defined as species which are produced in one step of a reaction and consumed in the other. It is important not to confuse an intermediate with a catalyst. Catalyst is consumed in one reaction and produced in one of the subsequent reactions.
Given the mechanism of this reaction, in the first step we produce
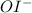 . Notice that the same species,
. Notice that the same species,
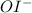 , is produced in the subsequent step 2. In this case,
, is produced in the subsequent step 2. In this case,
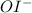 is the intermediate, as it follows the definition of an intermediate.
is the intermediate, as it follows the definition of an intermediate.
For your own information, iodide anion is a catalyst, as it's consumed in step 1 and reproduced in step 2.