Answer:
13.
a.Atomic number of cadmium is 48
b.Number of protons = 48
c.Mass number = 115 u
d.Number of neutrons=67
e.Isotopic Symbol=
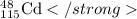
14. Elements which are isotopes are:
Step-by-step explanation:
13.
a.Atomic number(Z):The number of protons present in the nucleus of the atom. Every element have fixed characteristic atomic number which decides its chemical property.It is represented by Z
for example : hydrogen atomic no. is 1 ,He is 2
So Cadmium has attained 48 atomic number.
b.Number of protons = Atomic number of the element
The atomic number of Cadmium is 48. Hence
Number of protons in Cadmium=48
c.Mass number(A) = Total number of protons and neutrons present in the nucleus of the atom.It is represented by A
Mass number = number of proton + number of neutron.....(1)
Cadmium has mass number of 115
d.Using equation (1),
Number of neutron = mass number - number of proton
Number of protons in Cadmium=48
Cadmium has mass number = 115
Number of neutron = 115 - 48
=67
e.The format of symbolic representation of elements is given below
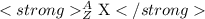
where X=symbol of element
Z= Atomic number
A= mass number
For Cadmium , X = Cd,Z = 48 , A =115
Symbol=
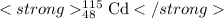
(Since this is isotope of cadmium hence it is isotopic symbol)
14.
Isotopes: These are the species which have same number of protons(or atomic number) but different number of neutrons(mass number).
- A ,C and E have same number of proton = 22 but different mass number. mass number :A =55,C=56 and E=58
So A ,C and E are isotopes
- B and D have same number of proton = 25 but different mass number
mass number: B=55 ,D=57
B and D are isotopes