Answer:
MOLAR MASS = 32 g/mol
Step-by-step explanation:
Condition of standard temperature and pressure(STP) are as follow:
Temperature = 273 K
Pressure = 1 atm (or 100000 Pa)
Here atm is atmosphere and Pa is Pascal
STP conditions arte used for measuring gas density and volume using Ideal Gas Law.Here 1 mole of ideal gas occupies 22.4 L of volume.
According toi Ideal Gas Equation :
PV = nRT
where P = pressure, n= number of moles, V = volume ,R= Ideal Gas Constant and T= temperature
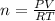
From question:
V=280 ml = 0.28 L
P = 1 atm
R=0.08205 L atm/K mol
T=273 K
Putting values in above formula :
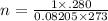
n = 0.0125 moles
Now
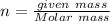
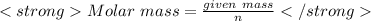
given mass = 0.4 g (given)
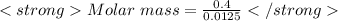
On solving we get:
Molar mass = 32 g/mol