Answer:
It is an example of single displacement reaction.
Step-by-step explanation:
Single replacement reaction:
A single element replaces another element in a compound.
For example,
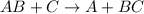
The given reaction is as follows.
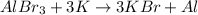
In the above reaction, aluminium bromide is dissociated into the
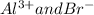 , these bromide ions are reacted with potassium atoms to form potassium bromide.
, these bromide ions are reacted with potassium atoms to form potassium bromide.
Here, only one element displaces therefore, it is an single displacement reaction.