An unknown gas is likely to be carbon dioxide if it turns limewater milky.
Option a
Step-by-step explanation:
Calcium Hydroxide also known as lime
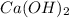 is a sparingly soluble compound which when dissolved in water produces an alkaline solution known as lime water. When a gas such as carbon dioxide
is a sparingly soluble compound which when dissolved in water produces an alkaline solution known as lime water. When a gas such as carbon dioxide
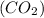 passes through this alkaline solution, it turns lime water milky due to the formation of an insoluble suspension of calcium carbonate
passes through this alkaline solution, it turns lime water milky due to the formation of an insoluble suspension of calcium carbonate
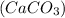 . This can be understood by the following chemical reaction-
. This can be understood by the following chemical reaction-
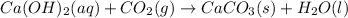
If an excess amount of
 gas is added in the solution, the following reaction takes place:
gas is added in the solution, the following reaction takes place:
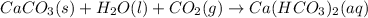
At this stage, the milkiness of the solution disappears since "calcium bicarbonate is soluble in water".