Answer: 8400 J
Step-by-step explanation:
The formula referenced in the question is:
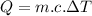
Where:
 is the thermal energy
is the thermal energy
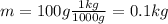 is the mass of the water sample
is the mass of the water sample
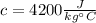 is the specific heat capacity of water
is the specific heat capacity of water
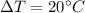 is the variation in temperature
is the variation in temperature
Solving:
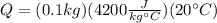
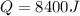 This is the thermal energy released
This is the thermal energy released