Answer:
The rate constant at 300 K , k = 0.0001386 s⁻¹
5000 s is the time required.
The activation energy is:- 124453.22 J/mol
Step-by-step explanation:
(i)
Half life is the time at which the concentration of the reactant reduced to half. So, 5000 s is the half life.
At 300 K
Half life expression for first order kinetic is:
Half life = 5000 s
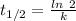
Where, k is rate constant
So,
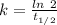
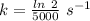
The rate constant, k = 0.0001386 s⁻¹
(ii)
As, stated above, Half life is the time at which the concentration of the reactant reduced to half. So, 5000 s is the time required.
(iii)
At 310 K , Half life = 1000 s
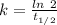
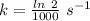
The rate constant, k = 0.0006931 s⁻¹
Using the expression,
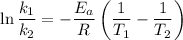
Wherem
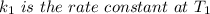
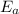 is the activation energy
is the activation energy
R is Gas constant having value = 8.314 J / K mol
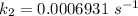
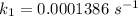
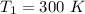
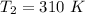
So,
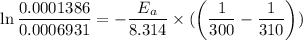
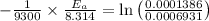
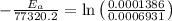
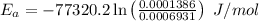
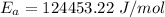
The activation energy is:- 124453.22 J/mol