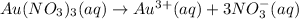Step-by-step explanation:
On dissolution of chromium(II) sulfate is water 2 ions of chromium(III) ion and and 3 sulfate ions are formed.
The balanced chemical equations of the dissolution of chromium (II) sulfate:
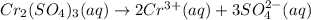
On dissolution of gold(III)nitrate is water 1 ions of gold(III) ions and and 3 ions of nitrate ions are formed.
The balanced chemical equations of the dissolution of gold(III) nitrate: