Answer:
0.0702J/g°C the specific heat capacity of the metal.
Explanation:m
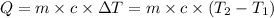
where,
Q = heat absorbed by metal = 186.75 J
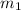 = Mass of metal= 19 g
= Mass of metal= 19 g
 = Initial temperature of metal =
= Initial temperature of metal =
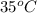
 =Final temperature of metal =
=Final temperature of metal =
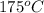
 = specific heat of metal= ?
= specific heat of metal= ?
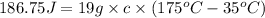
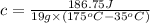
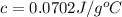
0.0702J/g°C the specific heat capacity of the metal.