Answer: The chemical equation is written below.
Step-by-step explanation:
Combustion reaction is defined as the chemical reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule.
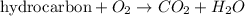
The chemical equation for the combustion of ethyl chloride follows:
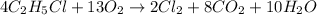
We are given:
When 4 moles of ethyl chloride is burnt, 5145 kJ of heat is released.
For an endothermic reaction, heat is getting absorbed during a chemical reaction and is written on the reactant side.
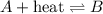
For an exothermic reaction, heat is getting released during a chemical reaction and is written on the product side
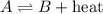
So, the chemical equation follows:
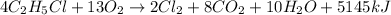
Hence, the chemical equation is written above.