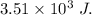Answer:
heat released is
Step-by-step explanation:
It is given that, heat capacity of calorimeter is, H =
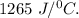
Also , initial temperature of calorimeter,
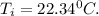
final temperature of calorimeter,
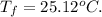
We know , Heat will release when it is positive.
So, heat released , Heat =
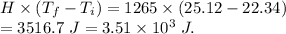 .
.
Since it is positive. Therefore, heat released is