Answer:
The structures are in attachment.
Step-by-step explanation:
a)
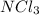
The given compound has one nitrogen atom and three chlorine atoms. To get the stable octet electronic configuration nitrogen acquire three electrons and three chlorine atom share one electron to the nitrogen atom.
b)

The given compound has one carbon atom and two oxygen atoms. To get the stable octet electronic configuration carbon acquire four electrons and two oxygen atoms are shared two electrons to the carbon atom.
c)
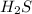
The given compound has one sulfur atom and two hydrogen atoms. To get the stable octet electronic configuration sulfur acquire two electrons and two hydrogen atoms shared two electrons to the sulfur atom.
d)
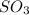
The given compound has one sulfur atom and three oxygen atoms. To get the stable octet electronic configuration sulfur acquire only two electrons and two oxygen atoms shared two electrons to the sulfur atom and remaining oxygen gets negative charge.
The structures are in attachment.