Answer:
The volume of the gas got doubled.
Step-by-step explanation:
Charle's law states that the volume of an ideal gas is direclty proportional to the absolute temperature.
Thus, V ∝ T
Or,
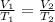
If the bottle is heated to the double of the temperature, the volume of the gas also gets doubled.