Answer:
no of moles of C6H14 required would be 3.07
Step-by-step explanation:
from the equation below we can see that 2 moles of C6H14 gives 12 moles of C02.
1 mole of C6H14 would give
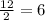 moles of CO2.
moles of CO2.
let the amount of C6H14 required to obtain 18.4 moles of CO2 be x
therefore x moles of C6H14 would produce 6x moles of CO2.
given 6x = 18.4
therefore x = 3.07 moles