Answer:
Percent yield is 54%
Step-by-step explanation:
The Grignard reaction is:
Mg + bromobenzene → phenylmagnesium bromide
Moles of Mg are:
2,3g×(1mol/24.3g) = 0.095 moles Mg.
Moles of bromobenzene are:
9,45mL×(1.495g/1mL)×(1mol/157.01g)= 0.0900 of bromobenzene.
The limiting reactant is bromobenzene. That means moles of phenylmagnesium bromide are 0.0900 moles.
To produce triphenyl carbinol you require:
2 phenylmagnesium bromide + methyl benzoate → triphenyl carbinol
Moles of methyl benzoate are:
5.12g×(1mol/136,15g) = 0.0376 moles of methyl benzoate.
The complete reaction of 0.0376 moles of methyl benzoate requires:
0.0376 moles of methyl benzoate×
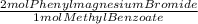 = 0.0752 moles of phenylmagnesium bromide. As you have 0.900 moles of phenylmagnesium bromide, limiting reagent is Methyl benzoate and moles of triphenyl carbinol are 0.0376. In grams:
= 0.0752 moles of phenylmagnesium bromide. As you have 0.900 moles of phenylmagnesium bromide, limiting reagent is Methyl benzoate and moles of triphenyl carbinol are 0.0376. In grams:
0.0376 moles of triphenyl carbinol×(260.33g/mol) = 9,79g of triphenyl carbinol -This is the teorethical yield-
Percent yield is the ratio of actual yield to the theoretical yield. That is:
5,3g / 9,79g ×100 = 54%
I hope it helps!