Answer: The mole percentage of helium in the mixture is 64.75 %
Step-by-step explanation:
To calculate the molar mass of mixture, we use ideal gas equation, which is:

Or,
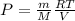
We know that:
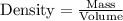
Rearranging the above equation:
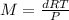
where,
M = molar mass of mixture = ?
d = density of mixture = 0.538 g/L
R = Gas constant =
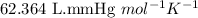
T = temperature of the mixture =
![25^oC=[25+273]K=298K](https://img.qammunity.org/2020/formulas/physics/high-school/h3swi627jfkpg7vx7in8p5pe35bz1gwehq.png)
P = pressure of the mixture = 721 mmHg
Putting values in above equation, we get:
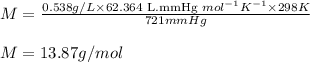
Molar mass of the mixture will be the sum of molar mass of each substance each multiplied by its mole fraction.
Let the mole fraction of Helium be 'x' and that of oxygen be '1-x'
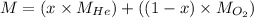
We know that:
Molar mass of helium = 4.00 g/mol
Molar mass of oxygen gas = 32g/mol
Putting values in above equation, we get:
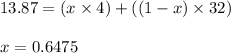
Mole fraction of helium in the mixture = 0.6475
Calculating the mole percentage of helium in the mixture:
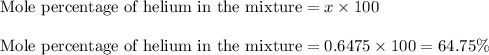
Hence, the mole percentage of helium in the mixture is 64.75 %