Answer:
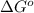 of the reaction is 1.7 kJ/mol.
of the reaction is 1.7 kJ/mol.
Hydrolysis of the pyrophosphate shifts the equilibrium of the reaction to the right, making the formation of acetyl-CoA energetically more favorable.
Step-by-step explanation:
The synthesis of acetyl CoA is as follows.
Form the given data,
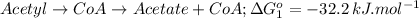
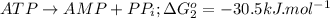
Total the above two steps we get,
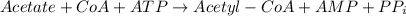
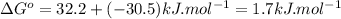
Therefore,
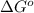 of the reaction is 1.7 kJ/mol.
of the reaction is 1.7 kJ/mol.
According to the Lechatelier's principle, a system at equilibrium always shows a tendency to regain the state of equilibrium., if equilibrium disturbed by an external factor only.
The enzyme inorganic pyrophosphotase catalyze the reaction.
The enzyme removes the ppi , one of the product in acetyl -CoA reaction.
This would disturb the equilibrium therefore, system try to regain the state of equilibrium. And the equilibrium shifted to the right side.
Therefore, Hydrolysis of the pyrophosphate shifts the equilibrium of the reaction to the right, making the formation of acetyl-CoA energetically more favorable.